The genetic heritage of the Denisovans may have left its mark on our mental health
A research team led by the Institute of Evolutionary Biology (IBE) and Pompeu Fabra University (UPF) has identified the most widespread genetic contribution by Denisovans to date.
The study reveals that the genetic variant observed, which affects zinc regulation, could have signified an evolutionary advantage in our ancestors’ adaptation to the cold.
The study, published by PLoS Genetics, also reveals that this genetic adaptation may have predisposed modern humans to neuropsychiatric disorders.
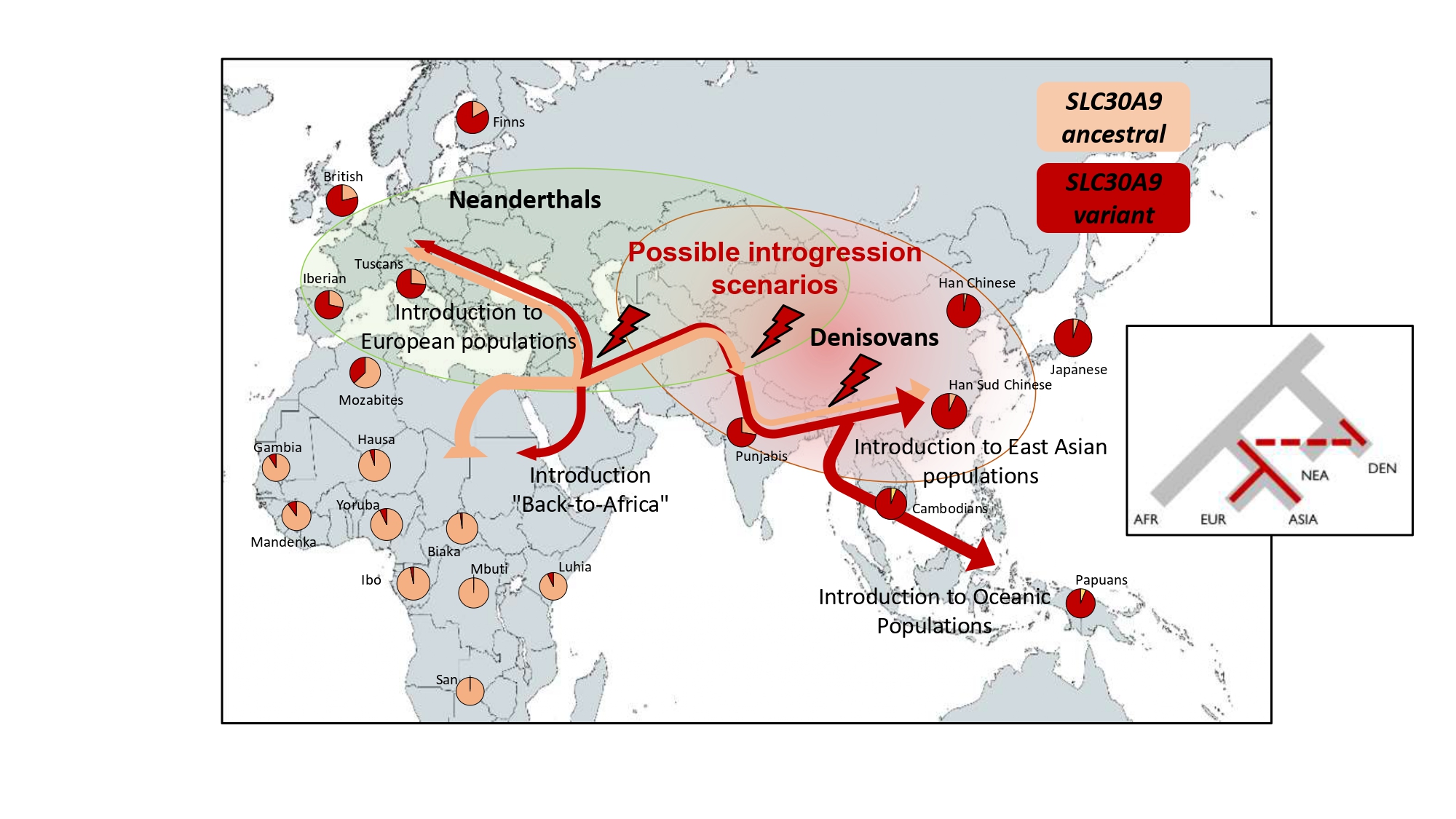
Modern humans left Africa some 60,000 years ago in the event known as “Out-of-Africa”. In Asia, they coincided with the Denisovans, and that encounter may have led to confrontations and collaborations, but also various crossbreeding. In fact, even today modern humans retain genetic variants of Denisovan origin in our genome, which are testimony to those initial interactions.
Now, a team led by the Institute of Evolutionary Biology (IBE), a joint centre of the Spanish National Research Council (CSIC) and Pompeu Fabra University (UPF), and by the UPF Department of Medicine and Life Sciences (MELIS), has identified one of the most widespread traces of the genetic heritage of the extinct Denisovans in modern humans. The teams of Elena Bosch, IBE principal investigator, and of Rubén Vicente, MELIS-UPF principal investigator, have discovered that this genetic adaptation helped ancestral populations of sapiens to adapt to the cold.
The variant observed, involved in zinc regulation and with a role in cellular metabolism, could also have predisposed modern humans to psychiatric disorders such as depression or schizophrenia.
Genetic variation in zinc regulation may have meant an evolutionary advantage
How adaptation has shaped current genetic diversity in human populations is a matter of great interest in evolutionary genetics.
Arising from this question, Elena Bosch’s team identified an adaptive variant among current human populations in a region of our genome that bears great similarity to the genome of an extinct ancestral population: the Denisovans.
“Through genomic analysis, we noted that the genetic variant observed came from our interbreeding with archaic humans in the past, possibly the Denisovans”, says Ana Roca-Umbert, co-first author of the study. The team has ruled out Neanderthal heritage, since these populations do not have this mutation.
“Apparently, the change was beneficial and proved a selective advantage for humans. As a consequence, this variation in the SLC30A9 gene was selected and has reached current populations”, adds Jorge Garcia-Calleja, co-first author of the study.
The Evolutionary Population Genetics Laboratory, directed by Bosch, wished to find out what changes are brought about by this genetic variation of Denisovan origin at the cellular level. “We discovered that this mutation surely had implications for the transport of zinc within the cell, and so we contacted Vicente's team”, recalls Elena Bosch, IBE principal investigator and co-leader of the study.
Zinc regulation: key to adapting to the cold
“Elena contacted me because her team had observed a change in an amino acid in a zinc transporter, which was very different between the populations of Africa and Asia today. From there, we started asking ourselves questions and looking for answers”, Rubén Vicente comments. His team, in the Biophysics of the Immune System group at the Laboratory of Molecular Physiology, undertook the technical challenge of studying the movement of intracellular zinc.
Zinc, an essential trace element for human health, is an important messenger that transfers both information from the outside to the inside of cells and between different cellular compartments. A lack of zinc causes growth, neurological and immune disorders, although “its regulation is still poorly studied due to the lack of molecular tools to follow the flow of zinc”.
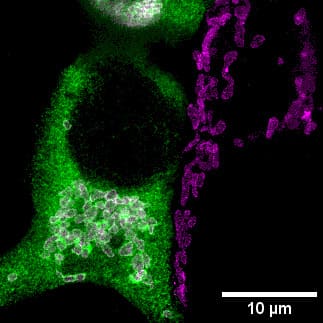
Vicente’s laboratory identified that the observed variant causes a new zinc balance within the cell, promoting a change in metabolism. By altering the endoplasmic reticulum and mitochondria of the cells, this variation causes a possible metabolic advantage to cope with a hostile climate. “The observed phenotype leads us to think of a possible adaptation to the cold”, Vicente asserts.
The Denisovan genetic heritage could affect the mental health of European and Asian populations
Zinc transport is also involved in nervous system excitability, and plays a role in people’s mental equilibrium and health.
The team points out that the variant found in this zinc transporter, which is expressed in all tissues of the body, is associated with a greater predisposition to suffering from some psychiatric diseases. These include anorexia nervosa, hyperactivity disorder, autism spectrum disorder, bipolar disorder, depression, obsessive compulsive disorder, and schizophrenia.
“In the future, expanding this study to animal models could shed light on this predisposition to suffering from mental illnesses”, Vicente notes.
The genetic variant has left a global mark, except in Africa
Although the variant was established in Asia as a result of interbreeding between Denisovans and sapiens, it also spread to European and native American populations. In fact, it is found in populations all over the planet, although, in the case of African populations, it is much less frequent.
The team points out that it is probably the Denisovan genetic adaptation to have the greatest geographical scope discovered to date. “For example, a variant in the EPAS1 gene inherited from the Denisovans allows adapting to life at altitude, but is found only in Tibetans. However, in our case, the impact extends to all populations outside Africa”, Bosch concludes.
Referenced study: Ana Roca-Umbert , Jorge Garcia-Calleja , Marina Vogel-González, Alejandro Fierro-Villegas, Gerard Ill-Raga, Víctor Herrera-Fernández, Anja Bosnjak, Gerard Muntané, Esteban Gutiérrez, Felix Campelo, Rubén Vicente, Elena Bosch. Human genetic adaptation related to cellular zinc homeostasis. Plos Genetics; DOI: https://doi.org/10.1371/journal.pgen.1010950
